Method Verification and Quality Control Procedures for Determination of phthalate
Introduction
The guidance in this Article describes the Method Verification and analytical quality control requirements to support the validity of data used for checking compliance with maximum residue limits (MRLs).
The key objectives are:
- To ensure the quality and comparability of analytical results.
- To ensure that acceptable accuracy is achieved.
- To ensure that false positives or false negatives are not reported.
Standards and Calibration Solutions
Identity and purity of standards:
- “Pure” standards of analytes should be of known purity. Each pure standard must be uniquely identified, the date of receipt recorded and an expiry date allocated if the supplier has not provided this.
- After the expiry date it must be replaced.
- Ideally, the identity of freshly acquired pure standards should be checked if the analytes are new to the laboratory.
| Name of Analyte |
Lot No. |
purity |
Date |
Date of expiry |
| DBP | XXXXX |
99.4 |
30/1/2021 |
Aug |
| BBP |
XXXXX |
98.5 |
30/1/2021 |
March |
| BIS-2EHP |
XXXXX |
99.4 |
30/1/2021 |
April |
| DNOP |
XXXXX |
99.2 |
30/1/2021 | March 2023 |
| DINP |
XXXXX |
99.9 |
30/1/2021 |
Aug 2023 |
| DIDP |
XXXXX |
99.0 |
30/1/2021 | MAY 2023 |
| Benzyl benzoate (IS) |
XXXXX |
99.5 |
30/1/2021 |
Jan 2023 |
Standard stock solution
Concentration of stock solution as follows.
|
Sr. No. |
Name of Analyte |
Amount taken for stock |
Final Volume of Solution
|
Concentration of stock solution mg/liter (ppm) |
|
1 |
DINP |
40.41 |
10 |
4036.959 |
|
2 |
DIDP |
14.81 |
10 |
1466.19 |
|
3 |
DBP |
29.12 |
10 |
2894.528 |
|
4 |
BBP |
21.24 |
10 |
2092.14 |
|
5 |
BIS-2EHP |
19.6 |
10 |
1948.24 |
|
6 |
DNOP |
24.3 |
10 |
2410.56 |
Internal standard calibration
solution
|
Sr. No. |
Name of Analyte |
Amount taken for stock |
Final Volume of Solution
|
Concentration of stock solution mg/liter (ppm) |
|
1 |
Benzyl benzoate |
12.2 |
10 |
1213.90 |
Working stock standard:
Preparation of linear concentrations of the standard Solution:
Injections were done of above serial dilutions in GCMS under following conditions:
Linearity
Calibration Curve
|
Sr. No. |
Name of Analyte |
For standard linearity R2 |
|
1 |
DBP |
0.997 |
|
2 |
BBP |
0.996 |
|
3 |
BIS-2EHP |
0.998 |
|
4 |
DNOP |
0.999 |
|
5 |
DINP |
0.996 |
|
6 |
DIDP |
0.995 |
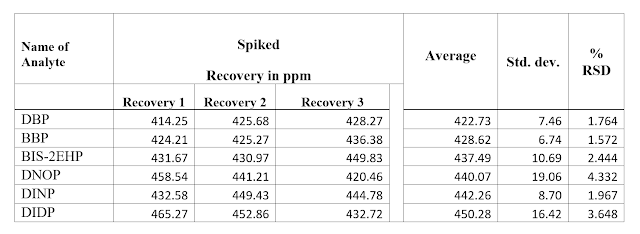
Repeatability & Recovery
DNOP & DIDP, DINP analyzed met the acceptability criteria of recovery (80-120%).
❤ Sample is spiked with three replicate analyses at the level of 500 mg/kg for DBP, BBP, BIS-2EHP &
at the level of 500 mg/kg for DIINP,
DIIDP prepared as per IS 9873-9:2017
& IS 9873-6:2017 SOP and injected in GCMS. Calculate standard deviation
and % RSD.
Above data indicates that DBP, BBP,
BIS-2EHP, DNOP & DIDP, DINP analyzed met the acceptability criteria of
recovery (80-120%).
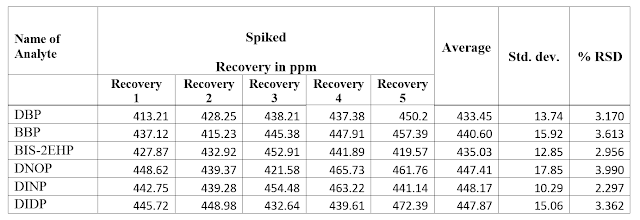
❤ Sample is spiked with replicate analyses at the level of 500 mg/kg for DBP, BBP, BIS-2EHP, DNOP
& at the level of 500 mg/kg for
DIINP, DIIDP prepared as per CPSC-CH-C1001-09.3/EN14372:2004
and injected in GCMS. Calculate standard deviation and % RSD.
Recovery as per CPSC-CH-C1001-09.3/EN14372:2004
Above data indicates that DBP, BBP,
BIS-2EHP, DNOP & DIDP, DINP analyzed met the acceptability criteria of recovery
(80-120%).
LOD & LOQ
Limit of Detection
❤ Limit of
Detection is the point at which a measured value is larger than the insecurity associated with it. It is the lowest concentration of analyte in a sample that
can be detected but not necessarily quantified.
❤ In
chromatography limit of detection is an amount that produces a peak with a
height at least 3 times that of the baseline noise level.
❤ Inject Standard Mixture of DBP, BBP, BIS-2EHP, and DNOP for five
replicate analyses at the level of 0.2ppm prepared as per method and injected
in GCMS. Calculate S/N.
| NAME OF ANALYTE |
S/N |
| DBP |
8.1 |
| BBP |
6.1 |
| BIS-2EHP |
8.4 |
| DNOP |
5.1 |
The Above data indicates that DBP, BBP, BIS-2EHP, DNOP analyzed by
each mode met the acceptability criteria of LOD (S/N>3).
❤ Inject Standard Mixture of DIDP, DINP for five replicate analyses at
the level of 2 ppm prepared as per method and injected in GCMS. Calculate S/N.
| Name of Analyte |
S/N |
| DINP |
4.4 |
| DIDP |
4.1 |
The Above data indicates of DIDP, DINP analyzed by each mode met the
acceptability criteria of LOD (S/N>3).
Limit of Quantification
❤ Limit of
Quantification is the minimum concentration of the analyte that can be quantified with acceptable precision should apply to the complete analytical
method. Variously defined but must be a value greater than the LOD.
chromatography limit of quantification is an amount that produces a peak with a
height 10 to 20 times that of the baseline noise level.
❤ Inject Standard Mixture of DBP, BBP, BIS-2EHP, and DNOP for five
replicate analyses at the level of 0.4 ppm prepared as per method and injected
in GCMS. Calculate S/N.
| Name of Analyte |
S/N |
| DBP |
18.8 |
| BBP |
12.4 |
| BIS-2EHP |
17.7 |
| DNOP |
14.3 |
The Above data indicates that DBP, BBP, BIS-2EHP, DNOP analyzed by
each mode met the acceptability criteria of LOQ (S/N>10).
❤ Inject Standard Mixture of DIDP, DINP for five replicate analyses at
the level of 5.0 ppm prepared as per method and injected in GCMS. Calculate
S/N.
| Name of Analyte |
S/N |
| DINP |
30.2 |
| DIDP |
12.4 |
The Above data indicates that DIDP, DINP analyzed by each mode met
the acceptability criteria of LOQ (S/N>10).
Conclusion
Based on above satisfactory verification of method with respect to
specification as per, IS-9873 part 9:2017/IS-9873 part 6:2017 &
CPSC-CH-C1001-09.3/EN 14372:2004 this is
to conclude that method is fit for the purpose & applied for the
determination of DBP, BBP, BIS-2EHP,
DNOP And DIDP, DINP.
|
Done by |
Checked by |
Approved by |
|
|
|
|
| Date | Date | Date
|